Candidozyma auris – an invisible threat in hospitals
Candida auris, now officially renamed Candidozyma auris, is a yeast fungus first identified in Japan in 2009 that has since spread globally. Unlike most other fungi, it is remarkably resilient: it can survive on hospital surfaces for weeks, resists many disinfectants, and spreads easily. While harmless for healthy individuals, it can cause life-threatening infections in immunocompromised patients. Hospitals face a serious challenge, as C. auris can spread rapidly among already weakened patients and is often resistant to multiple antifungal drugs, leaving limited treatment options.
Its clinical impact is severe. While other Candida species mainly cause skin or mucosal infections, C. auris can enter the bloodstream and trigger a so-called fungemia — a systemic infection that can lead to organ failure and death. Intensive care units are particularly affected, as patients there often require catheters, ventilation, or invasive procedures. To make matters worse, C. auris is difficult to identify using standard laboratory tests, which often misclassify it as another Candida species. This delay in accurate diagnosis means delayed treatment — time that can be crucial for survival.
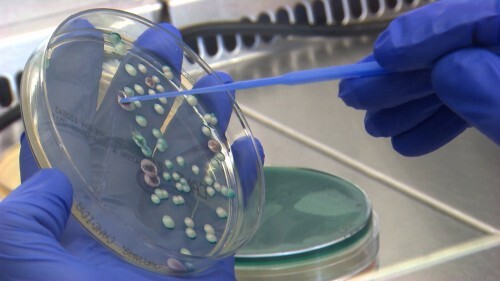
According to the European Centre for Disease Prevention and Control (ECDC), more than 4,000 cases of Candida auris have been reported across EU/EEA countries between 2013 and 2023. In 2023 alone, 18 countries documented 1,346 cases — a sharp rise compared to previous years. Southern and Eastern Europe are most affected: Spain, Italy, Greece, and Romania are already facing endemic situations where the fungus has become established. Germany has also seen repeated outbreaks that were difficult to contain. Clearly, C. auris has become a growing public-health issue, not an isolated event.
The situation in the U.S. underlines the urgency. In Michigan alone, as of September 1, 2025, authorities recorded 1,944 cases of Candida auris. Of these, 468 were clinical infections, while others represented colonization detected through screening. The numbers prove that this pathogen is no longer a medical rarity — its a global threat.
Scientists are now exploring new treatment and prevention approaches. One promising candidate is lactoferrin, a natural iron-binding protein found in human milk. Laboratory research suggests lactoferrin can inhibit C. auris growth in vitro, and when combined with existing antifungal drugs, its effect is amplified. However, clinical data from animal or human studies are still pending.
Until effective therapies become available, prevention remains the best strategy against Candida auris. Hospitals must maintain strict hygiene protocols, disinfect surfaces thoroughly, and regularly screen high-risk patients. International collaboration and consistent case monitoring are essential to control its spread. One thing is certain: Candida auris is here to stay — and the medical world must adapt quickly to meet this invisible threat.